how many valence electrons does bi have|Valence Electrons Chart for All Elements : Cebu Element Bismuth (Bi), Group 15, Atomic Number 83, p-block, Mass 208.980. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main . Rinakrakan patalikod ang Sweet pinay baby. Tuwang tuwa ng ma Creampie ang Pekpek ng Tsinitang Tisay. Creampie ang masarap na puke ni Inday Sara. Masungit parin si bebe. Adik sa tsupaan tong si Mary Jane. Sarap pumatong ni baby love sheet swerte swerte ni .
PH0 · Valence electrons (video)
PH1 · Valence Electrons Chart for All Elements
PH2 · Valence Electrons
PH3 · Determine valence electrons using the periodic table
PH4 · Complete Electron Configuration of Bismuth (Bi, Bi3+, Bi5+)
PH5 · Complete Electron Configuration of Bismuth (Bi,
PH6 · Bismuth Valence Electrons (And How to Find them?)
PH7 · Bismuth (Bi)
PH8 · Bismuth
PH9 · 10.6: Valence Electrons
El video de bella poarch que todos queremos tener / 10 min. 10 min AbneyZimmer - 561.9k Views - 720p. Morena , bella , nalgona 8 sec. 8 sec Sophia5361 - 720p. Pounding Bella Bellz Big Ass 5 min. 5 min Bangbros Bubble Butts - 12.4M Views - 720p. BANGBROS - Bella Bellz in Miami bitches! 8 min. 8 min Bangbros Network - 9.3M Views -
how many valence electrons does bi have*******Mar 23, 2023 Element Bismuth (Bi), Group 15, Atomic Number 83, p-block, Mass 208.980. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main . This electron configuration shows that the bismuth ion(Bi 5+) has five shells and the last shell has eighteen electrons and it achieves a stable electron configuration. Bismuth atom exhibit +3, +5 .Bismuth is a Post Transition Metal element. It is part of group 15 (nitrogen family). Know everything about Bismuth Facts, Physical Properties, Chemical Properties, Electronic configuration, Atomic and Crystal .how many valence electrons does bi have Valence Electrons Chart for All Elements Bismuth has 5 valence electrons because there are 5 electrons present in the outermost shell of the Bismuth (Bi) atom. Now let’s see how you can easily find the .You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 .The number of electrons in a neutral atom is equal to the atomic number, or number of protons, in an atom. Valence electrons are the electrons which live in the outermost . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; .
How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is reached. Neon, with its configuration ending in .
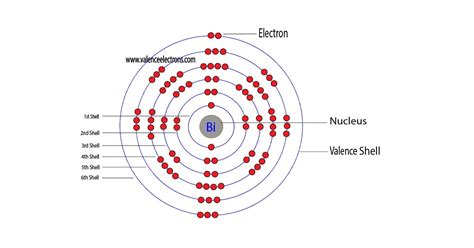
Method 1: From the Periodic Table. To find out the valence electrons of Bismuth, you have to see the position of bismuth in the periodic table. More specifically, you have to see the group wise position of Bismuth element in the periodic table. From the above image, you can see that the Bismuth (Bi) is present in the group 15 of periodic table.
Overview Electron configuration. The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. Thus, the number of valence electrons that it may have . (i.e., all group 1 elements have 1 valence electron, all group 2 elements have 2 valence electrons, skip the transition metals. then, all group 13 elements have 3 valence electrons, all group 14 elements have 4 valence electrons, and so on up to group 18 elements). Since bismuth is in group 15, it has 5 valence electrons.

Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. B: 1s 2 2s 2 2p 1 (there are three electrons on the highest occupied energy level n=2) In fact, the number of valence electrons goes up by one for each step across a . Step 4: Find Valence Electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Thus, bromine has seven valence electrons. Valency of Bromine (Br)
In the case of titanium, it has an atomic number of 22, which means it has 22 electrons. The electronic configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. The valence electrons of an atom are the electrons in the outermost energy level or shell.
The electron configuration shows that the beryllium atom has acquired the electron configuration of helium. That is, in this case, the valence of the beryllium ion (Be +2) is +2. Since the last shell of a beryllium ion has two electrons, the valence electrons of beryllium ion (Be +2) are two.
We know that arsenic atoms have a total of thirty-three electrons. The electron configuration shows that the first shell of arsenic has two electrons, the second shell has eight electrons, the 3rd shell has eighteen electrons and the 4th shell has five electrons. Therefore, the number of electrons per shell of arsenic is 2, 8, 18, 5.
how many valence electrons does bi haveHow many valence electrons does a Bismuth atom have? Bismuth has 5 valence electrons. Bismuth has 83 electrons out of which 5 valence electrons are present in the 6s2 6p3 outer orbitals of atom. What is .
For this, nickel ion (Ni 2+) has a total of sixteen valence electrons. Again, the nickel atom donates two electrons in the 4s orbital and an electron in the 3d orbital to convert nickel ion (Ni 3+ ). Ni – 3e .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .When determining the number of outer-shell electrons, we are always focusing on the s, and p block, as the number can go up to 8. Bismuth is located in Group 15 of the Periodic Table of Elements.The valence electrons are the electrons in the outermost electron shell of an atom. That is why elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration s2p6.
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .Valence Electrons Chart for All Elements How many valence electrons in Bi? Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, .
How Many Valence Electrons Does Bromine (Br) Have? By Farhan Sadik Posted on March 20, 2023 June 30, 2024 Updated on June 30, 2024. Bromine is the 35th element in the periodic table and the 3rd element in group 17. Bromine is a halogen element and its symbol is ‘Br’.
Secure desktop login for current Charles Schwab clients
how many valence electrons does bi have|Valence Electrons Chart for All Elements